Now Reading: Why Spilled Coffee Forms Ring-Shaped Stains
-
01
Why Spilled Coffee Forms Ring-Shaped Stains
Why Spilled Coffee Forms Ring-Shaped Stains
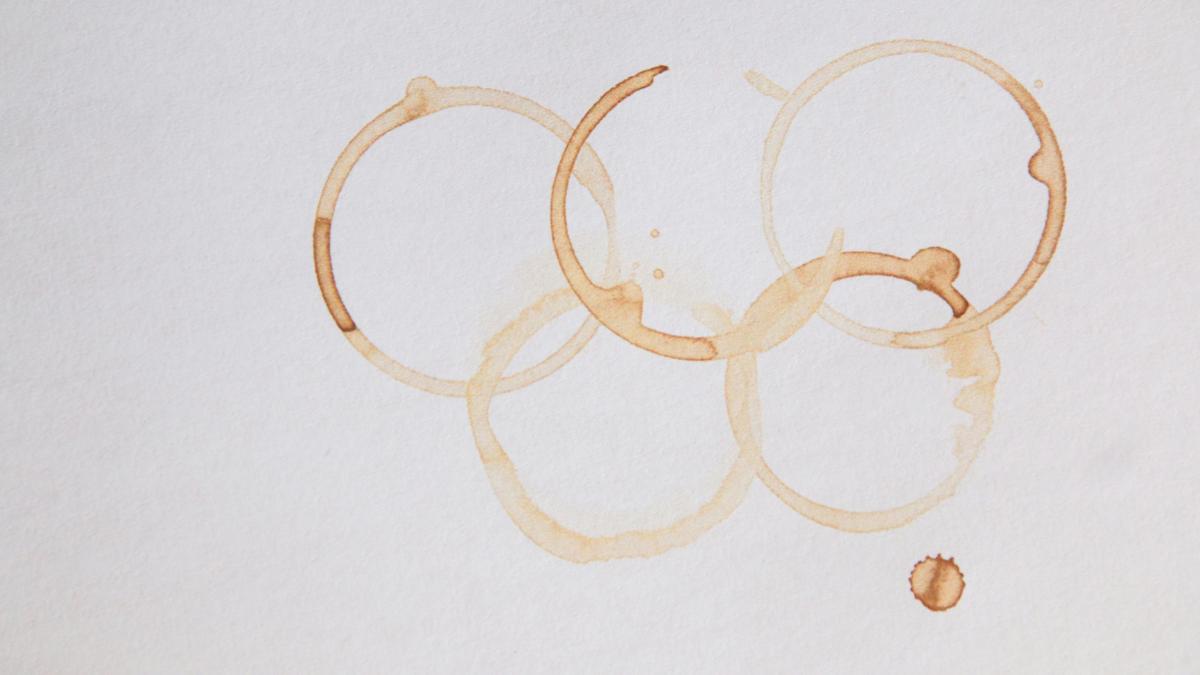
Speedy Summary
- When a drop of coffee spills and dries, it ofen leaves a dark ring with a pale center, known as the “coffee-ring effect.”
- This pattern is caused by how the liquid evaporates and how suspended particles in coffee move.
- The edge of the drop is typically “pinned” to the surface, where evaporation happens faster compared to the thicker center.
- A capillary flow develops as liquid moves outward from the middle to replenish water lost at the rim, carrying suspended particles that build up at the edge.
- The pattern can be altered or weakened by factors like fluid circulation changes (caused by temperature or concentration differences),addition of surfactants or polymers,reduced evaporation rates,or freeing up pinned edges.
- Particle shape also affects patterns; elongated grains tend to resist movement toward edges and create a more uniform film.
Indian Opinion Analysis
The “coffee-ring effect” provides an insightful example of physics in everyday phenomena while holding broader implications for industries like material science and biotechnology. In India’s thriving research landscape, understanding such fluid dynamics could lead to innovations in inkjet printing technologies or medical diagnostic tools relying on particle deposition patterns. Encouraging interdisciplinary studies at universities can amplify discoveries linking basic physical principles to practical applications for Indian markets.